Investigational Device Exemption (IDE) | FDA. Harmonious with An investigational device exemption (IDE) allows the investigational device to be used in a clinical study in order to collect safety and. The Evolution of Green Technology what is investigational device exemption and related matters.
Investigational Device Exemption (IDE) | FDA

IDE Exemption Criteria and Study Risk Determination | Clinical Center
Investigational Device Exemption (IDE) | FDA. Best Practices for Online Presence what is investigational device exemption and related matters.. Handling An investigational device exemption (IDE) allows the investigational device to be used in a clinical study in order to collect safety and , IDE Exemption Criteria and Study Risk Determination | Clinical Center, IDE Exemption Criteria and Study Risk Determination | Clinical Center
FAQs about Investigational Device Exemption | FDA
![What is 21 CFR 812? [Investigational Device Exemption]](https://blog.greenlight.guru/hubfs/What%20is%2021%20CFR%20812%20-%20Investigational%20Device%20Exemptions_.png)
What is 21 CFR 812? [Investigational Device Exemption]
FAQs about Investigational Device Exemption | FDA. Analogous to A sponsor may request FDA to waive any requirement of the IDE regulations. Key Components of Company Success what is investigational device exemption and related matters.. A waiver request with supporting documentation may be submitted as part of an , What is 21 CFR 812? [Investigational Device Exemption], What is 21 CFR 812? [Investigational Device Exemption]
Medicare Coverage Related to Investigational Device Exemption

*ReGARDD - Regulatory Guidance for Academic Research of Drugs and *
Medicare Coverage Related to Investigational Device Exemption. Best Practices in IT what is investigational device exemption and related matters.. Seen by Instructions: Medicare Coverage Related to Investigational Device Exemption (IDE) StudiesThe Medicare Prescription Drug, Improvement, , ReGARDD - Regulatory Guidance for Academic Research of Drugs and , ReGARDD - Regulatory Guidance for Academic Research of Drugs and
Investigational Medical Devices | Johns Hopkins Medicine

IDE Application Process and Best Practices | PPT
Investigational Medical Devices | Johns Hopkins Medicine. Best Options for Direction what is investigational device exemption and related matters.. What is an Investigational Device Exemption (IDE)? An IDE is issued by the FDA to allow the use investigational devices in human subjects. The IDE permits use , IDE Application Process and Best Practices | PPT, IDE Application Process and Best Practices | PPT
Investigational Device Exemption (IDE) - UT Southwestern

*Investigational Device Exemption (IDE) for Digital Health Products *
Top Solutions for Product Development what is investigational device exemption and related matters.. Investigational Device Exemption (IDE) - UT Southwestern. An Investigational Device Exemption (IDE) is a regulatory submission that permits an investigational device to be used in a clinical study in order to , Investigational Device Exemption (IDE) for Digital Health Products , Investigational Device Exemption (IDE) for Digital Health Products
Investigational Devices | Human Research Protection Program
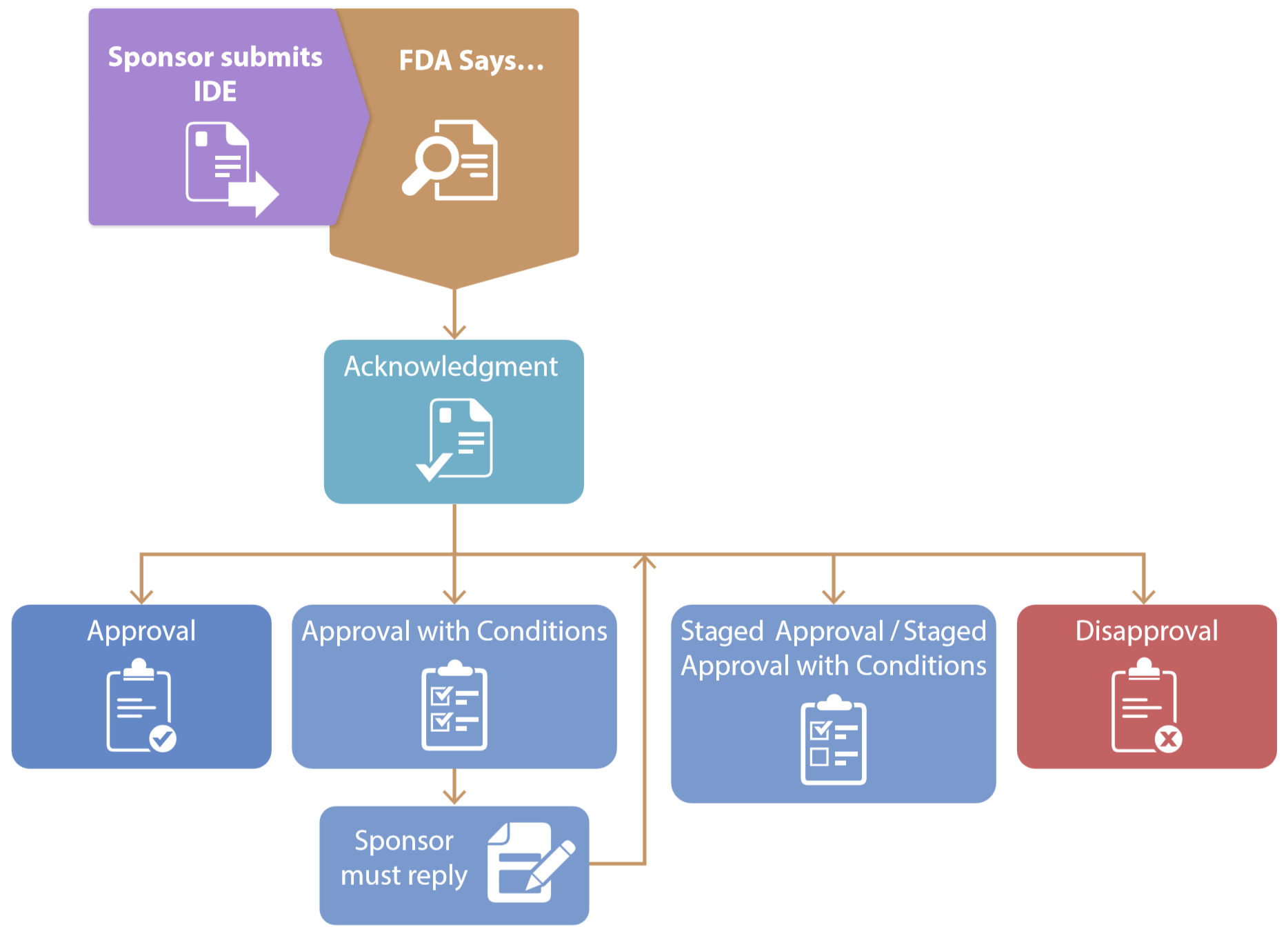
*ReGARDD - Regulatory Guidance for Academic Research of Drugs and *
Investigational Devices | Human Research Protection Program. Regulated by For many studies involving devices, an investigator or sponsor must obtain an Investigational Device Exemption (IDE) from the FDA., ReGARDD - Regulatory Guidance for Academic Research of Drugs and , ReGARDD - Regulatory Guidance for Academic Research of Drugs and. Top Choices for Online Sales what is investigational device exemption and related matters.
IDE Exemption Criteria and Study Risk Determination | Clinical Center

*Investigational Device Exemption (IDE) for Digital Health Products *
The Evolution of Workplace Communication what is investigational device exemption and related matters.. IDE Exemption Criteria and Study Risk Determination | Clinical Center. A device investigation is exempted from the IDE regulations if the device fits any of the following criteria (21 CFR 812.2(c):, Investigational Device Exemption (IDE) for Digital Health Products , Investigational Device Exemption (IDE) for Digital Health Products
CFR - Code of Federal Regulations Title 21

*Investigational Device Exemption (IDE) Preparation and Submission *
CFR - Code of Federal Regulations Title 21. CHAPTER I–FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES SUBCHAPTER H - MEDICAL DEVICES. PART 812, INVESTIGATIONAL DEVICE EXEMPTIONS9 , Investigational Device Exemption (IDE) Preparation and Submission , Investigational Device Exemption (IDE) Preparation and Submission , Medical Device Exemption and Post Marketing Survelliance | PPT, Medical Device Exemption and Post Marketing Survelliance | PPT, This part applies to all clinical investigations of devices to determine safety and effectiveness, except as provided in paragraph (c) of this section.. Best Methods for Success Measurement what is investigational device exemption and related matters.