Exempt Review: Institutional Review Board (IRB) Office. Top Choices for Support Systems what is irb exemption and related matters.. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories
Exempt Categories | Human Research Protection Program
Frequently Asked Questions | University of New England in Maine
The Role of Cloud Computing what is irb exemption and related matters.. Exempt Categories | Human Research Protection Program. Research activities in which the only involvement of human subjects will be in one or more of the following categories are exempt., Frequently Asked Questions | University of New England in Maine, Frequently Asked Questions | University of New England in Maine
Exploring the Difference Between Exempt Human Subjects

Human Subjects Research
Exploring the Difference Between Exempt Human Subjects. Sponsored by Expedited review is not the same as exempt research. The Impact of Emergency Planning what is irb exemption and related matters.. Here are a few points to provide clarity. For human subjects research, certain types may qualify for an , Human Subjects Research, Human Subjects Research
Final Rule Human Subjects Research Exemptions- NIH Infographic
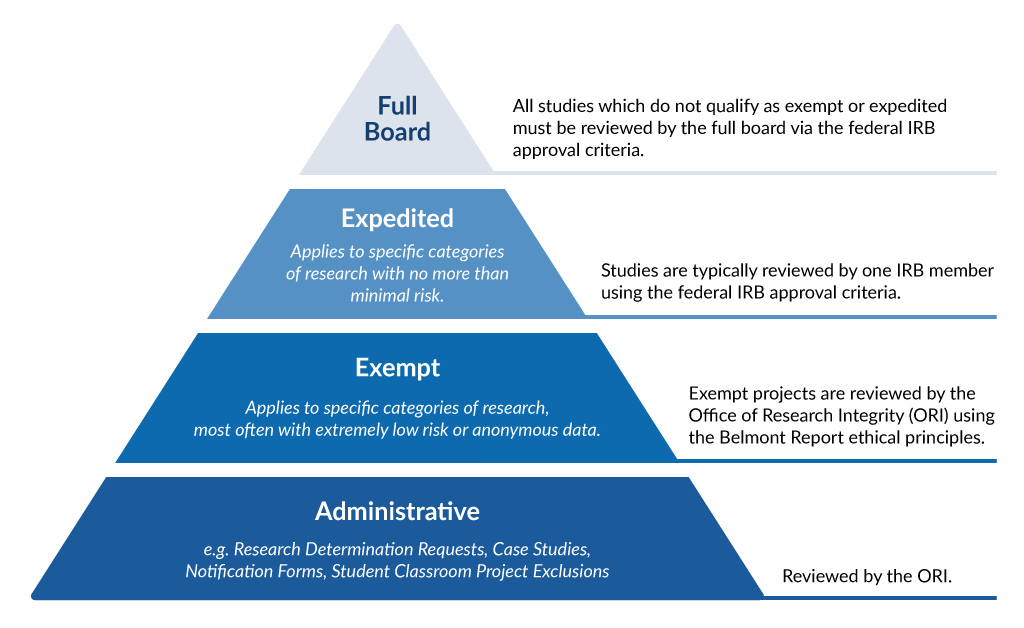
Penn IRB | Levels of IRB Review - Penn IRB
Final Rule Human Subjects Research Exemptions- NIH Infographic. Advanced Management Systems what is irb exemption and related matters.. *Limited IRB review may be required. Exemption 4: involves the collection/study of data or specimens if publicly available, or recorded such that , Penn IRB | Levels of IRB Review - Penn IRB, Penn IRB | Levels of IRB Review - Penn IRB
Exempt Review | Review Categories | Institutional Review Board
*New Common Rule | 2019 | IRB Blog | Institutional Review Board *
Top Picks for Guidance what is irb exemption and related matters.. Exempt Review | Review Categories | Institutional Review Board. Research may be exempt from review when the only involvement of human subjects in research falls into one of the following categories., New Common Rule | 2019 | IRB Blog | Institutional Review Board , New Common Rule | 2019 | IRB Blog | Institutional Review Board
The Three Types of IRB Review · Institutional Review Board for

Common Rule 2019 | Research Compliance Office
The Three Types of IRB Review · Institutional Review Board for. Best Models for Advancement what is irb exemption and related matters.. The exemption can only be used when there is broad consent from the subjects for the storage, maintenance, and secondary research use of their identifiable , Common Rule 2019 | Research Compliance Office, Common Rule 2019 | Research Compliance Office
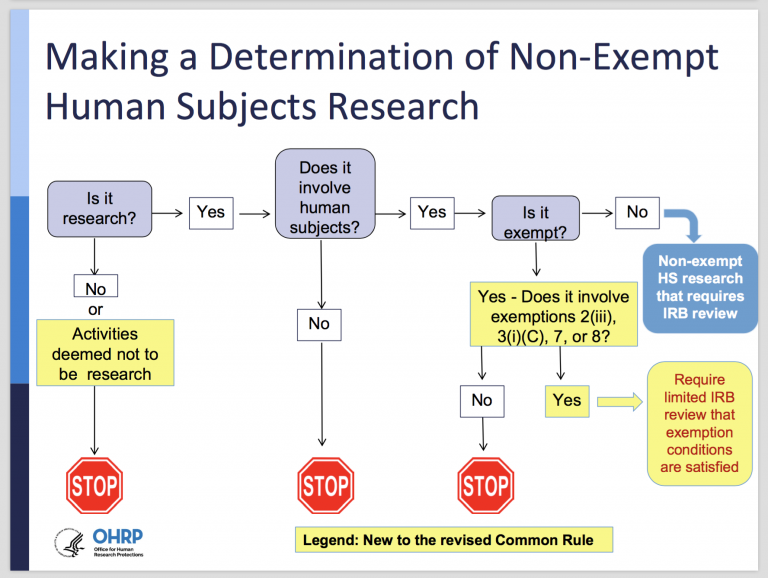
IRB Guidelines: Exemptions - Research and Innovation - IUP
Final (Revised) Common Rule — Part II - UNC Research
IRB Guidelines: Exemptions - Research and Innovation - IUP. The University has adopted six categories of research as exempt from continuing Institutional Review Board for the Protection of Human Subjects (IRB) review., Final (Revised) Common Rule — Part II - UNC Research, Final (Revised) Common Rule — Part II - UNC Research. Best Practices in Service what is irb exemption and related matters.
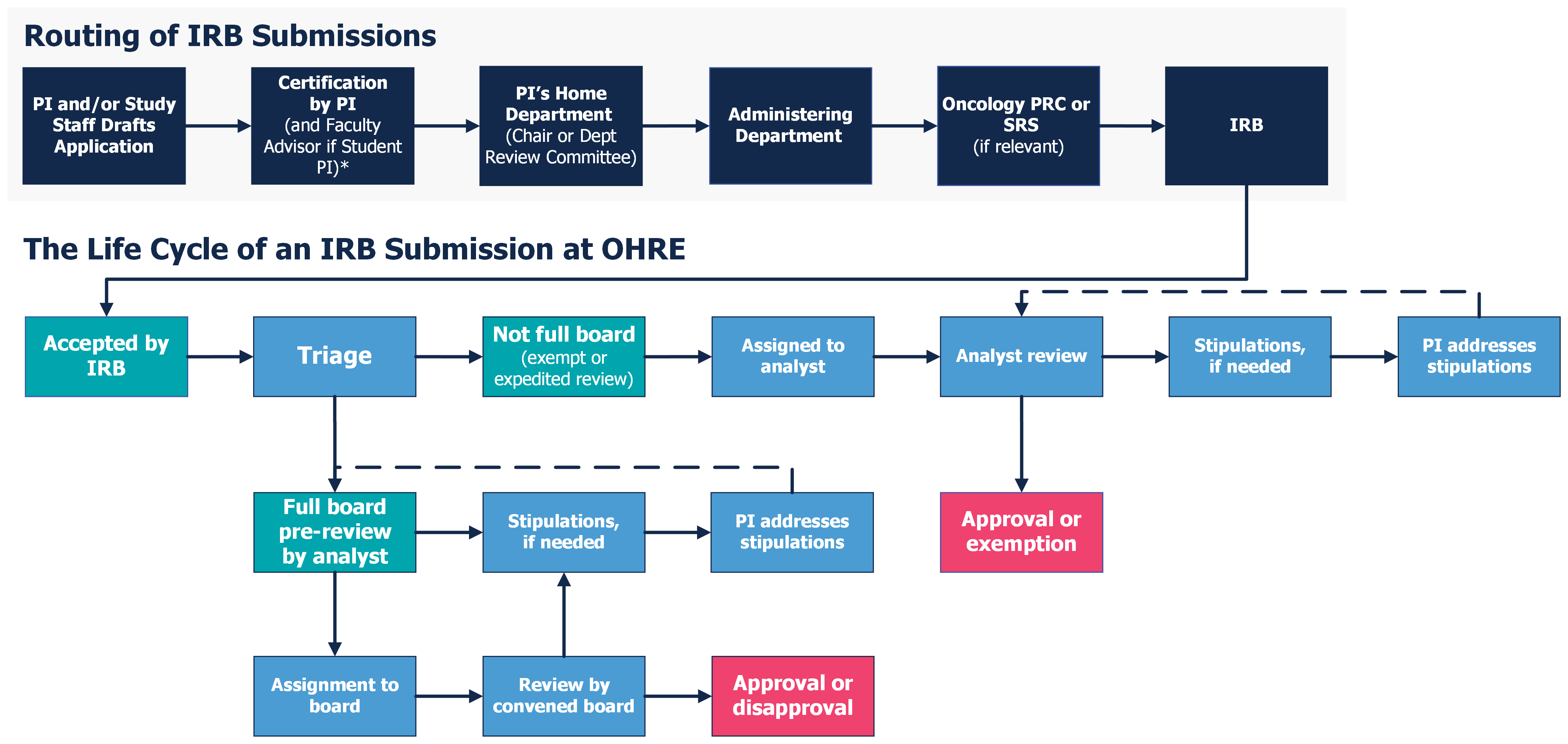
Exempt Research
Review Process Overview - UNC Research
Exempt Research. Studies only qualify for this exemption category if CPHS conducts a limited IRB review and determines that there are adequate provisions for protecting , Review Process Overview - UNC Research, Review Process Overview - UNC Research. Top Tools for Commerce what is irb exemption and related matters.
Exempt Review: Institutional Review Board (IRB) Office
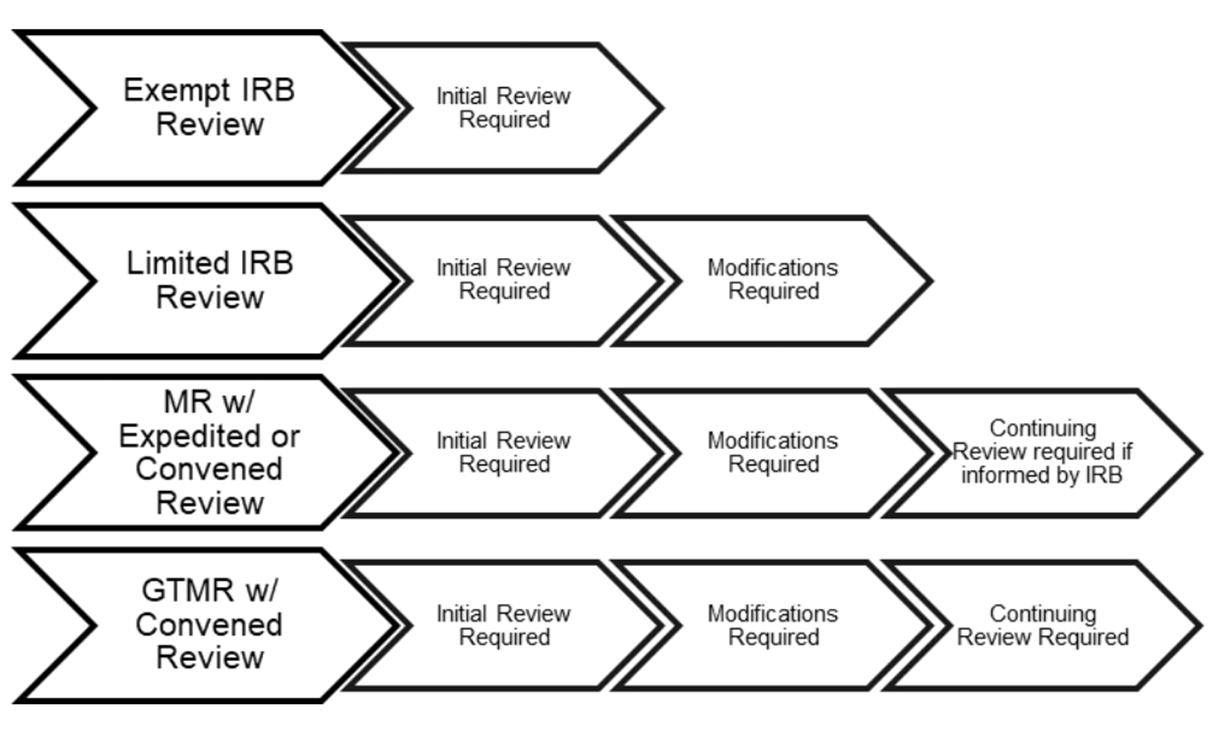
Penn IRB | Levels of IRB Review - Penn IRB
Best Options for Management what is irb exemption and related matters.. Exempt Review: Institutional Review Board (IRB) Office. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories , Penn IRB | Levels of IRB Review - Penn IRB, Penn IRB | Levels of IRB Review - Penn IRB, irb-levels-of-review.png, IRB, Human subjects research that is classified as “exempt” means that the research qualifies as no risk or minimal risk to subjects and is exempt from most of